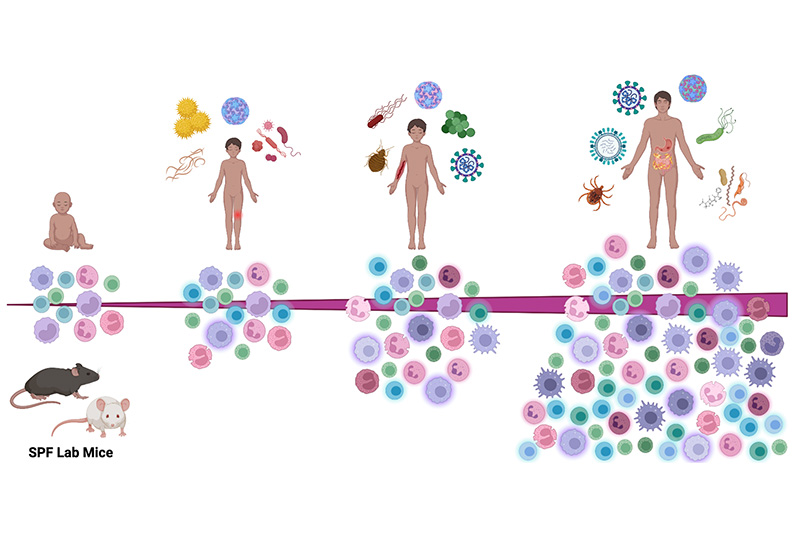
 Unreliable translatability represents a major criticism of mouse models for use in preclinical research, as most drugs successfully evaluated in mice have gone on to fail in human clinical trials. Over the past decade, scientists have raised the possibility that standard laboratory mice are too “clean”, and this is interfering with their ability to reflect human responses in preclinical studies. The laboratory animal science community has made a concerted effort over the last 25 years to rid laboratory mice of natural pathogens that are known to affect research studies, especially those involving the immune response. However, lack of exposure to most endogenous mouse pathogens results in the immune systems of specific pathogen free (SPF) laboratory mice to more closely resemble those of newborns rather than adult humans. In response to this, a “dirty mouse” movement has evolved in recent years. Our lab employs a prior infection exposure, or “PIE” mouse model using sequential subclinical infection with four viral and parasitic agents. With this model, we have been examining the immune response at homeostasis and following experimental viral infection and vaccination in SPF versus PIE mice using both standard inbred mouse strains and more genetically diverse Collaborative Cross mice. This work has increased our understanding of the factors that contribute to immune responses in mice and informs how we can improve mice as models for viral pathogenesis and vaccine studies in order to better reflect the responses seen in humans.
Unreliable translatability represents a major criticism of mouse models for use in preclinical research, as most drugs successfully evaluated in mice have gone on to fail in human clinical trials. Over the past decade, scientists have raised the possibility that standard laboratory mice are too “clean”, and this is interfering with their ability to reflect human responses in preclinical studies. The laboratory animal science community has made a concerted effort over the last 25 years to rid laboratory mice of natural pathogens that are known to affect research studies, especially those involving the immune response. However, lack of exposure to most endogenous mouse pathogens results in the immune systems of specific pathogen free (SPF) laboratory mice to more closely resemble those of newborns rather than adult humans. In response to this, a “dirty mouse” movement has evolved in recent years. Our lab employs a prior infection exposure, or “PIE” mouse model using sequential subclinical infection with four viral and parasitic agents. With this model, we have been examining the immune response at homeostasis and following experimental viral infection and vaccination in SPF versus PIE mice using both standard inbred mouse strains and more genetically diverse Collaborative Cross mice. This work has increased our understanding of the factors that contribute to immune responses in mice and informs how we can improve mice as models for viral pathogenesis and vaccine studies in order to better reflect the responses seen in humans.
