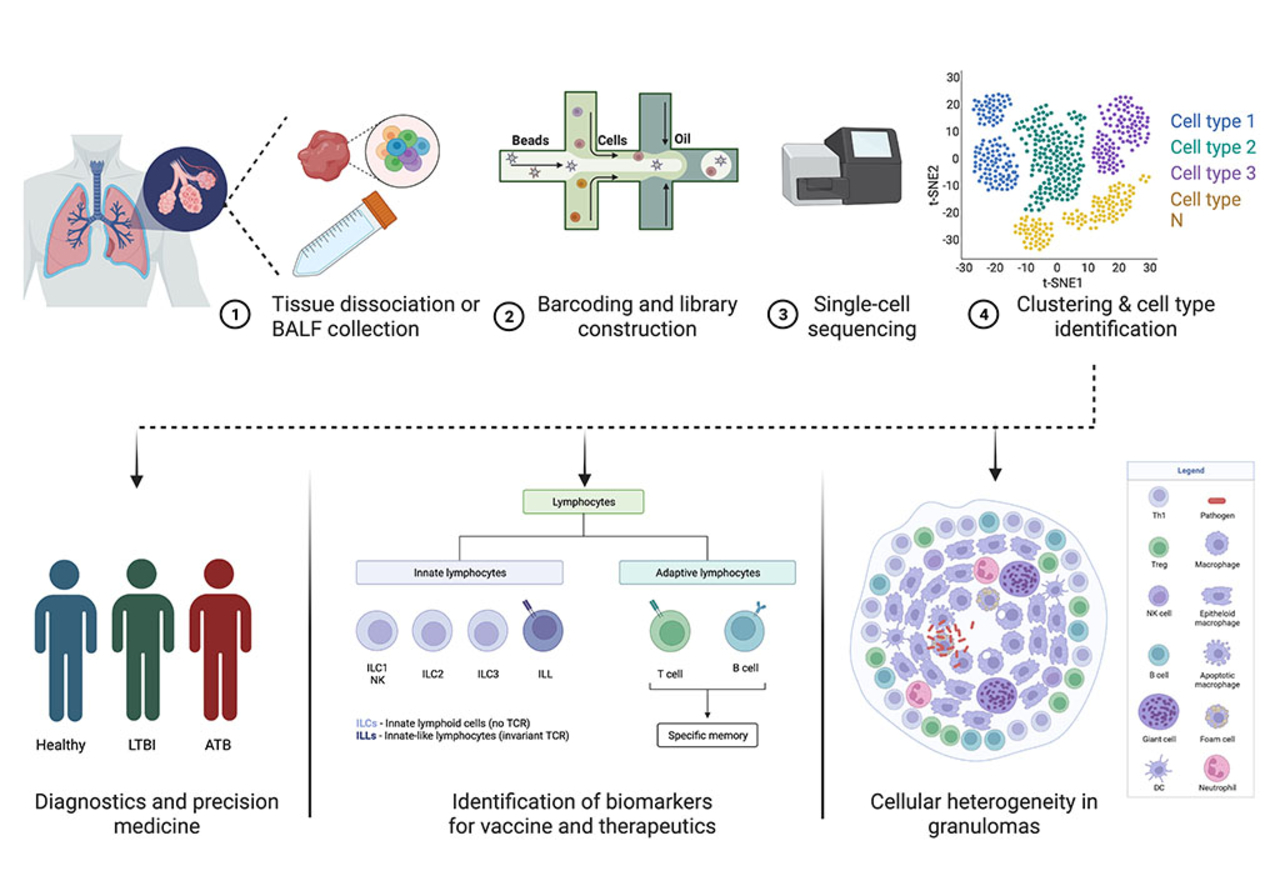
Overview
We have showed that initiation of antiretroviral therapy (cART) at peak viremia led to i) better control of viral replication, ii) significantly reduced immune activation in the lung vasculature and iii) reduced macrophage turnover in the lung of macaques. However, skewed CD4+ T effector memory responses persisted and new TB lesions formed despite cART initiation as early as 2 weeks post-SIV. Understanding why skewed effector responses persist despite the control of viral replication by cART is critical to forming a causal link between immune activation and T cell function. Similar to humans, SIV differentially impacts alveolar macrophages (in BAL) and interstitial macrophages in lungs. We hypothesize that cART is unable to rescue the impaired T- effector function in the lungs of Mtb/SIV co-infected macaques despite reducing the macrophage turnover resulting in worsening of disease condition and LTBI reactivation. Our research utilizes scRNA-seq technology to identify the specific lineage markers in the interplay between macrophages and CD4+ T cells that remain impaired despite cART. This is critical to developing interventions to control immune activation and prevent LTBI reactivation upon HIV co-infection.