Immunology
Biology of Metabolite Antigen Presentation
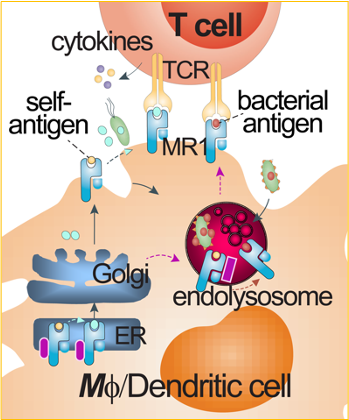 Conventional CD4+ and CD8+ T cells are activated by peptide antigens presented by major histocompatibility complex (MHC) class I and class II molecules, named human leukocyte antigens (HLA) in humans. Peptide antigen presentation has shaped a classical paradigm in T cell activation and vaccine development. Endogenous peptides (e.g., mammalian or viral peptides) and exogenous peptides (bacterial peptides) can differentially utilize secretory, cross-presentation, and endocytic pathways to be loaded onto HLA proteins and activate conventional CD4+ and CD8+ T cells. As unconventional T cells, mucosal-associated invariant T (MAIT) cells and lipid-reactive T cells respond to metabolite and lipid antigens presented by MHC class I-like molecules MHC-related protein 1 (MR1) and CD1, respectively. The non-peptidic metabolite or lipid antigen presentation can involve different sets of accessory proteins and alternative pathways for T cell activation. Our lab will characterize metabolite antigen presentation pathways for MAIT cell activation.
Conventional CD4+ and CD8+ T cells are activated by peptide antigens presented by major histocompatibility complex (MHC) class I and class II molecules, named human leukocyte antigens (HLA) in humans. Peptide antigen presentation has shaped a classical paradigm in T cell activation and vaccine development. Endogenous peptides (e.g., mammalian or viral peptides) and exogenous peptides (bacterial peptides) can differentially utilize secretory, cross-presentation, and endocytic pathways to be loaded onto HLA proteins and activate conventional CD4+ and CD8+ T cells. As unconventional T cells, mucosal-associated invariant T (MAIT) cells and lipid-reactive T cells respond to metabolite and lipid antigens presented by MHC class I-like molecules MHC-related protein 1 (MR1) and CD1, respectively. The non-peptidic metabolite or lipid antigen presentation can involve different sets of accessory proteins and alternative pathways for T cell activation. Our lab will characterize metabolite antigen presentation pathways for MAIT cell activation.
Representative Publications
Biology of Unconventional T cell Responses
Unconventional T cells, particularly MAIT cells, bear innate-like features of activation and responses. MAIT cells can respond to microbial infections in much rapid kinetics through recognizing conserved metabolite antigens presented by MR1 protein. This activation mechanism is independent of individual HLA genetic variability in humans. The relatively high frequency of MAIT cells in various tissue sites in humans provides an ideal model and supports the significance of understanding the roles of MAIT cells in diseases and interventions. We are characterizing the unique immune responses of MAIT cells and other unconventional T cells to understand their functions in infectious diseases, particularly tuberculosis and comorbidities, and in some cancers. Immunology assays, transcriptomics, targeted gene mutation, and animal vs. human tissue models with chemical intervention will be used in our studies.
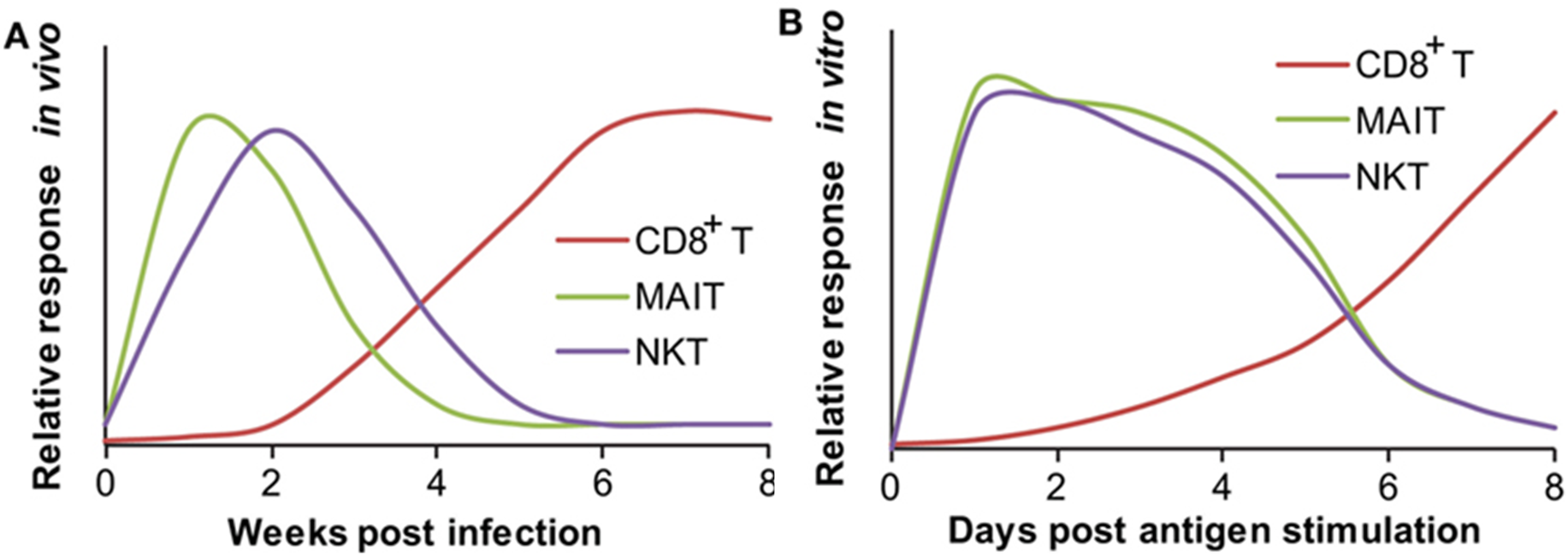
RESPONDING KINETICS OF MUCOSAL-ASSOCIATED INVARIANT T (MAIT) AND NATURAL KILLER T (NKT) CELLS. (A) In vivo responding kinetics were hypothesized based on the presence of mycobacterial antigen-specific CD8+ T cells in lung tissues, the ability of MAIT cells to inhibit Bacillus Calmette–Guérin (BCG) growth in lung tissues, and the ability of transferred iNKT to inhibit M. tuberculosis growth in lung tissues. (B) In vitro-responding kinetics were estimated according to the acquisition of cytolytic function by CD8+ T cells upon in vitro peptide stimulation, cytokine production by polyclonal MAIT cells upon stimulation with BCG-infected macrophages, and cytokine production by tetramer-isolated human polyclonal NKT cells upon antigen-specific activation.
