Our Research
About Our Research
Despite viral suppression by anti-retroviral drugs, chronic inflammation/immune activation persists in HIV-infected patients and increases their risks of developing non-AIDS related illnesses such as cardiovascular disease, neurological dysfunction, enteropathy, metabolic disease, etc. Dr. Mohan and his research team have made significant contributions to the understanding of epigenetic mechanisms underlying HIV induced gastrointestinal dysfunction and more importantly, how these changes can be inhibited/reduced using anti-inflammatory cannabinoids. His research focuses on several different aspects of HIV/SIV pathogenesis. His laboratory performs both basic and translational studies using the premier SIV-infected macaque model of HIV/AIDS to:
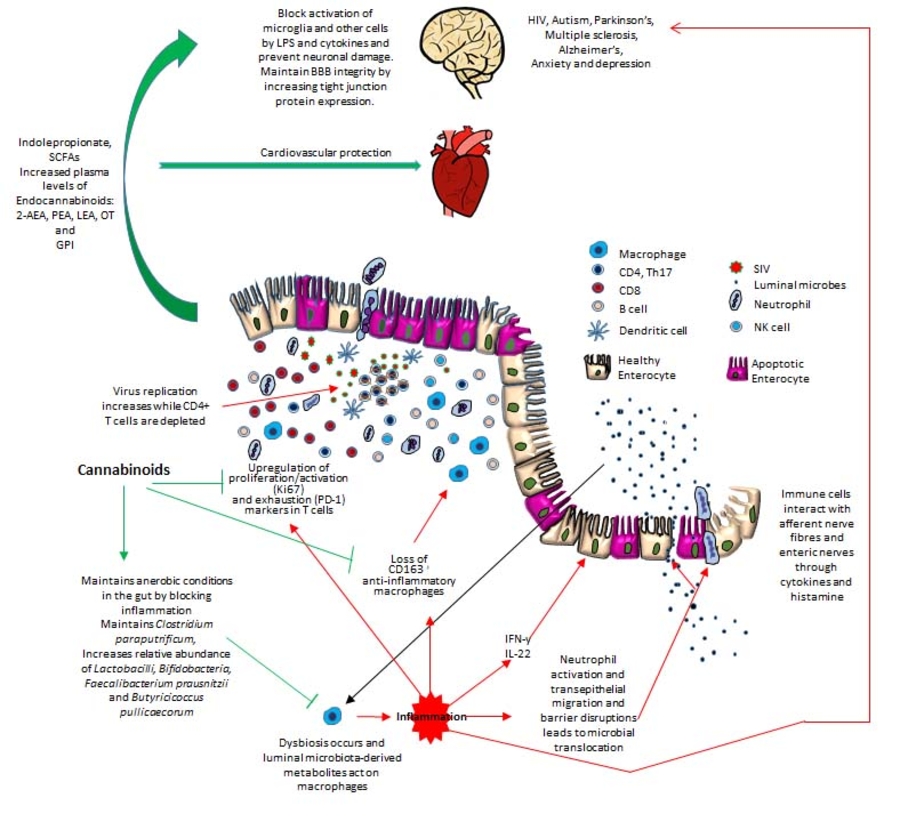
- Identifying receptor mediated and other downstream epigenetic mechanisms associated with the anti-inflammatory effects of cannabinoids. Current research efforts are to identify the role of cannabinoid receptor 2 and peroxisome proliferator activated receptor gamma using specific agonists in the in vivo setting. Specific downstream epigenetic mechanisms (molecular) that we are actively exploring include DNA methylation and small and long non-coding RNAs.
- Investigating the anti-fibrotic effects of cannabinoids and related compounds and identifying specific molecular mechanisms underlying the ability of cannabinoids to inhibit collagen deposition in lymph nodes.
- Identifying proinflammatory pathways and networks associated with HIV-associated neuroinflammation and explore the potential of long-term low dose cannabinoids to suppress neuroinflammation in the setting of ART.
- Evaluate the effect of cannabinoids on the secretion and composition of extracellular vesicles in HIV/SIV infection. This is a NIH funded study focused on HIV/SIV induced gastrointestinal epithelial dysfunction and lymphoid fibrosis.
- Drug abuse, including cocaine, continue to be a significant co-morbidity of HIV infection. Drug usage is associated with risky behaviors and poor adherence to antiretroviral drugs, which leads to increased incidences of HIV infection and poor long-term prognosis of disease course. In addition to negative behavioral manifestations, drugs of abuse have been shown to directly impact the immune system leading to a neuroinflammatory state in the central nervous system. Planned studies will investigate the effects of cocaine on viral replication, neuroinflammation, immune dysfunction and dysbiosis.
- Investigate immune activation in adipose tissue, an HIV/SIV reservoir, and how long-term low dose cannabinoids can modulate proinflammatory gene expression within the adipose tissues.
- Develop the HIV-associated cardiovascular disease model in rhesus macaques and determine the potential of cannabinoids to decrease inflammation and cardiometabolic disease.
- Exploring cannabinoids as a viable host directed therapeutic strategy to treat Mtb/HIV coinfection. The primary focus is to inhibit immune activation and the at the same time inhibit the indoleamine 2-3-dioxygenase-1 pathway and make more tryptophan available for conversion to serotonin and microbiota synthesized indole derivatives.
- Epigenetic regulation of intestinal epithelial barrier (lncRNA and DNA methylation).
- Explore the gut-brain-axis and what kind of modulations cannabinoid treatment can provide in the context of HIV/SIV infection.
Laboratory Collaborations
Mohan’s lab current collaborators include: Dr. Chioma Okeoma (Stonybrook University in New York), Dr. Siddappa Byrareddy (University of Nebraska, Omaha), Drs. Deepak Kaushal, Smita Kulkarni, Diako Ibrahimi and Xavier Alvarez (Texas Biomed/SNPRC), Dr Jerzy Szablowski (Rice University) and Dr. Jason Kimata (Baylor College of Medicine).
Main Technologies and Methods Used
- T cell, B cell, and monocyte/macrophage immunology
- Intestinal epithelial cell biology
- Primary lymph node fibroblast cell culture and biology
- Transcriptomics (microRNA/mRNA/lncRNA) including RNAseq and microarrays
- Spatial transcriptomics and ATAC-seq
- Gene/microRNA overexpression, knockdown and RT-qPCR.
- Luciferase reporter and RNA pull down assays
- Immunofluorescence/Confocal microscopy
- Whole animal Positron emission and computerized tomography (PET/CT) imaging
- Immunoprecipitation/Western Blotting
- Flow cytometry
- Reduced representation and targeted bisulfite sequencing



