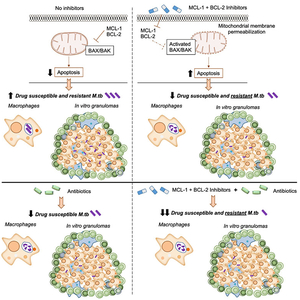
Alveolar macrophages (AMs) are sentinels in the lung which clear inhaled agents, including allergens and microbes; yet do not efficiently clear M. tuberculosis (M.tb). We found that a transcription factor highly expressed in AMs (PPARγ) that is critical for M.tb growth inside human macrophages, induces expression of MCL-1 (family member of anti-apoptotic BCL-2 proteins. Further, MCL-1 limit apoptosis and contributes to M.tb ability to grow intracellularly in human macrophages (Arnett et al. PLoS Pathog 2018). Apoptosis, whereby cells die with intact membranes, limits inflammatory responses and contributes to antigen presentation and T cell activation during M.tb infection (Arnett and Schlesinger Immunity 2021). We are investigating MCL-1 and other anti-apoptotic BCL-2 proteins as targets for host-directed therapy and have found that combinations of inhibitors that are FDA-approved and in clinical trials for cancer therapy significantly reduces growth of drug susceptible and resistant M.tb in human macrophages and a pre-clinical human granuloma model. Since these inhibitors are efficacious in rodent cancer models, and safety and activity studies are underway in cancer clinics, we expect that re-purposing them for TB treatment should translate more readily and rapidly to the clinic than generation of new compounds. Thus, this host-directed therapy approach could signify a breakthrough for TB treatment.
