We study the host-pathogen interaction to improve our understanding regarding how the host immune system responds to M.tb infection and how this is manipulated by M.tb to propagate infection, to help identify targets for host-directed therapy (HDT). There are many benefits to HDT, including that HDTs:
- are expected to work against both drug susceptible and resistant TB
- provide a treatment option that the pathogen is unlikely to develop resistance to
- shorten treatment duration
- boost the immune response
- ameliorate pathology associated with severe disease.
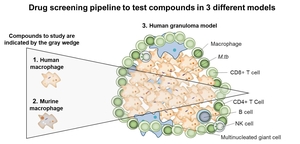
HDTs could be used in conjunction with antibiotics and could become an important tool in the fight against infectious diseases like TB. Our pipeline for testing HDTs for TB consists of first testing efficacy in human and murine macrophages, then testing those compounds in a more complex human granuloma model that we developed, to identify compounds that can penetrate and retain activity in macrophages and the unique granuloma environment.
