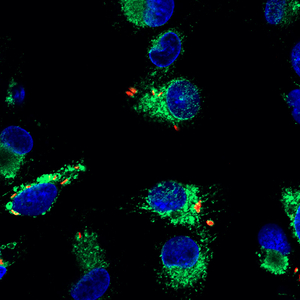
Mycobacterium tuberculosis (M.tb) is the causative agent of tuberculosis (TB) which kills 8,000 people daily worldwide. M.tb is a host-adapted intracellular pathogen that can hijack host signaling pathways to favor its own survival. Early interactions between M.tb and macrophages can dictate the outcome of infection. A long-term goal of the Schlesinger lab is to identify intracellular “master regulators” of inflammation and metabolic intermediates that dictate human immune responses to M.tb infection as a strategy to identify potential targets for host-directed therapy (HDT) for TB.
The mannose receptor is a major phagocytic receptor for M.tb on human macrophages, first identified by the Schlesinger lab. Our work has shown that the mannose receptor is important for activation of the nuclear receptor PPARγ, which is critical for bacterial growth in the human macrophages. Mannose receptor is also critical for limiting phagolysosomal fusion and promotion of bacterial growth. Future studies will further delve into the role of mannose receptor in M.tb pathogenesis.
We have identified the transcription factor cyclic AMP response element binding protein (CREB) as one such master regulator. CREB is critical for intracellular growth of M.tb in human macrophages and is key for various immune evasion strategies used by M.tb including restriction of NF-kB nuclear translocation and inhibition of phagolysosomal fusion. We seek to gain further understanding of the signaling pathways regulated by CREB in human macrophages during M.tb infection.
