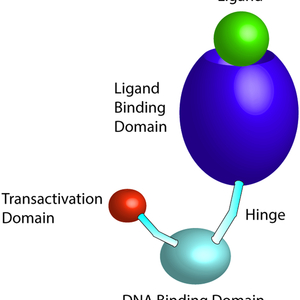
Nuclear receptors are a class of transcription factors that act as master regulators of many different cellular and immunological processes, such as metabolism, cytokine production, and programmed cell death. Nuclear receptors are classified as conventional, orphan, or adopted orphan depending on whether an endogenous ligand has been identified. Upon binding a ligand, nuclear receptors can translocate from the cytoplasm to the nucleus to exert their transcription factor effects, although same nuclear receptors may already be present in the nucleus. It is known that different nuclear receptors may be permissive or restrictive to M.tb growth, even within the same family. Nuclear receptors are bona fide drug targets and an attractive target for host-directed therapies (HDTs). Our lab has studied the nuclear receptor peroxisome proliferator-activated receptor gamma (PPARγ) and shown that counteracting PPARγ’s effectors, such as MCL-1 and BCL2, induces apoptosis in macrophages and significantly decreases M.tb growth. Ongoing projects in the lab center on PPARγ and other nuclear receptors that are important for the host response to M.tb.
